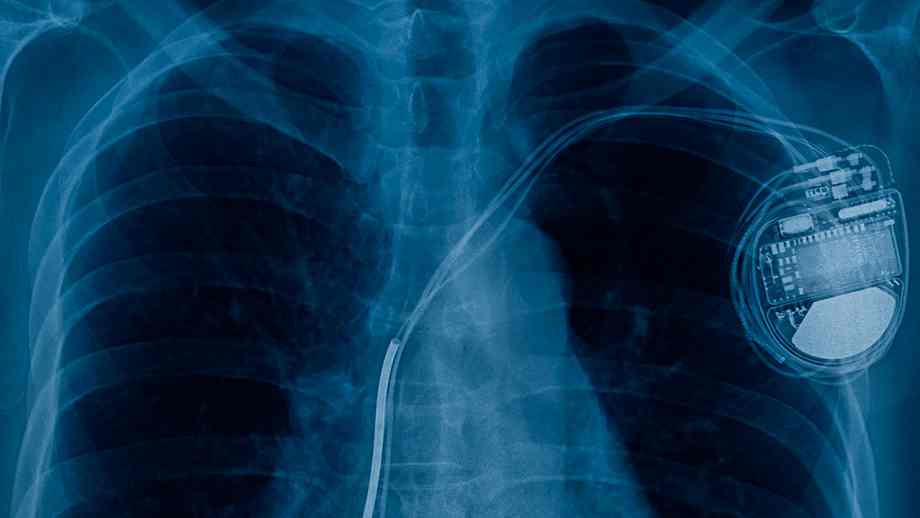
The units, made by Abbott, work to detect when the guts is thrashing too slowly, after which ship alerts to the mind to make it beat on the right tempo. If there may be {an electrical} brief within the machine, it might spur mistaken info, influence battery life, lose pacing operate or require substitute.
PENCE RECEIVES PACEMAKER AFTER SLOW HEART RATE: WHAT TO KNOW
“If the machine is unable to ship pacing, sufferers could expertise sluggish or irregular heartbeat, fainting, shortness of breath, tiredness, dizziness or discomfort,” the FDA stated, on its recall discover. “Moreover, shorter battery life and machine life could result in an extra pacemaker substitute process earlier than anticipated. Lastly, if the system doesn’t relay correct info through telemetry, medical suppliers could not know to offer therapy.”
Abbott first notified sufferers of the difficulty in March, and stated there was no suggestion to switch the machine if there was no proof of a difficulty as a result of low incident charge. It did suggest substitute for units that skilled sudden battery depletions or finish of service warnings, or in sufferers who skilled “a medical influence.”